More than half of the most recent new dietary ingredient (NDI) notifications provided by the Food and Drug Administration (FDA) in the last six months were filed without comment, but the rate of notifications reported received by the agency is dramatically lower than previously observed for any six month period since the initial six months in 1995 when the agency first began accepting and filing notifications.
AHPA recently discussed an unusual number of notifications submitted to FDA through the first months of 2017 (see Technical issues prevent FDA from evaluating majority of recent NDI notifications), but an equally unusual circumstance appears to have occurred during the remainder of the 2017 calendar year, with FDA resources reporting 7 new notifications received between those discussed in our most recent report and January 1, 2018. During the second half of the year proper, from July 1, 2017 through December 31, 2017, only 3 notifications were reported by agency personnel. The AHPA NDI Database has been updated with the latest analysis and records associated with these newly available notifications and AHPA staff are investigating the cause of the recent drop in NDI notifications released by FDA.
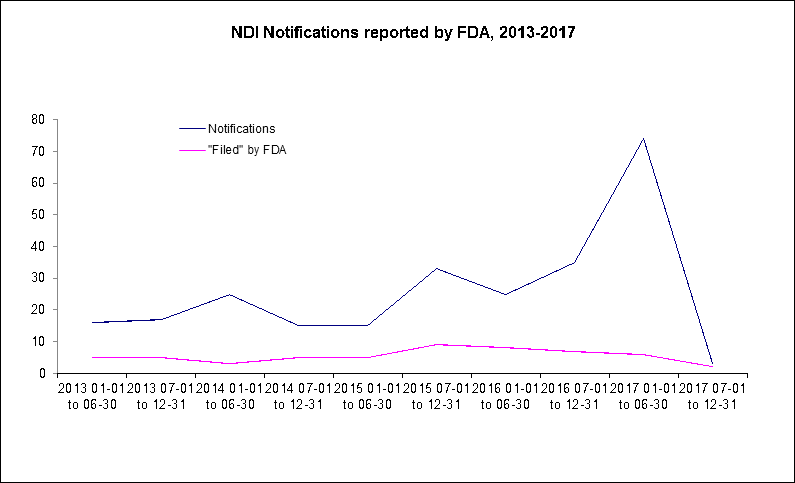
Among these seven notifications most recently released by FDA and added to the NDI Database, four of these, 57 percent, were filed by FDA without substantive comment, with AHPA's NDI Database connecting three to previously submitted notifications from 2016 and 2013, one of which was previously filed with an objection by the agency. The remainder included two notifications which were reported by the agency as not describing a dietary ingredient, and one which was incomplete. The details of these and other NDI notifications are available through AHPA's NDI Database.
"The fact that more than half of these recent NDI notifications were filed without comment is an impressive feat from industry members who work hard to help the agency understand the basis of expected safety of these innovative ingredients," said AHPA Chief Information Analyst Merle Zimmermann, Ph.D. "In the most recent notifications, the most striking objection resulted from unwarranted disease claims included with a submission of an otherwise well characterized dietary ingredient, leading the agency to recommend that if the product was intended to treat a disease condition, it would need to be processed through the drug notification process."
This type of marketing claim is cautioned against in industry resources such as AHPA's claims substantiation webinar. Research tools such as AHPA's NDI database can provide a reference as to recent actions taken by the agency as well as tools to ensure a submission clearly communicates the information required under 21 CFR 190.6.
AHPA's NDI Database provides instant access to written summaries of the salient points in FDA's responses to NDI notifications (when available) and information on the dates of submission and processing. The database enables users to browse by report number or search notifications by company and ingredient, including common or Latin names for botanicals. An "Outcome Statement" is also provided to help users quickly understand FDA responses, including issues that resulted in FDA comments.
Companies that want to use a dietary ingredient not marketed in the U.S. before Oct. 15, 1994 are required to submit an NDI notification explaining why the ingredient is reasonably expected to be safe. This notification must be submitted at least 75 days before the dietary ingredient is introduced into interstate commerce.
FDA does not "approve" or "disapprove" NDI notifications. Instead, the agency generally provides one of several responses, including but not limited to: (1) letter of acknowledgement without objection; (2) letter listing deficiencies that make the notification incomplete; (3) objection letter raising safety concerns based on information in the notification or identifying gaps in the history of use or other evidence of safety; and (4) letter raising other regulatory issues with the NDI or dietary supplement (e.g., the NDI is not a dietary ingredient as defined by regulation or the product is excluded from the definition of "dietary supplement" under current regulations because it is not intended for ingestion).
AHPA NDI Resources