Data from the most recent FDA inspections of dietary supplement facilities conducted in 2016 and 2017 show FDA continues to inspect an increasing number of facilities annually and the industry continues to get better at communicating compliance to FDA inspectors.
AHPA Chief Information Analyst Dr. Merle Zimmermann will present a detailed overview of AHPA's extensive FDA cGMP inspection data and specific examples of FDA observations at AHPA's upcoming, two-part webinar.
FDA inspected 610 dietary supplement facilities in fiscal year (FY) 2017, up from roughly 475 in FY 2016 and FY 2015. FDA has inspected a total of 2,137 unique dietary supplement facilities (including 184 foreign facilities) of companies that market supplements in the U.S. between January 2010 and September 2017. Among the 1,953 domestic inspections, 52 percent (1,015) of the most recent FDA inspections resulted in no recorded observations or FDA Form 483s.
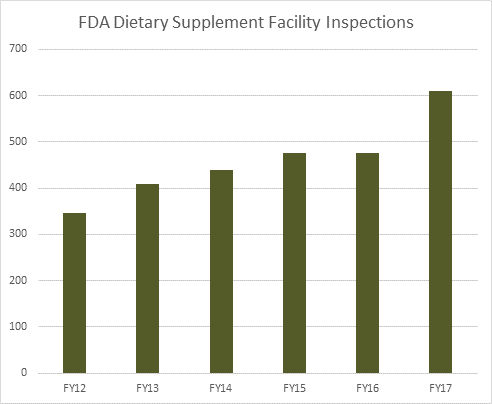
While the data suggests that FDA inspectors are finding more facilities in compliance with 21 CFR 111, some sections of the cGMP requirements still frequently appear in FDA Form 483s. The graph below documents the most common sections that appeared in FDA inspection observations concluded in FY 2016 and the first two quarters of FY 2017.
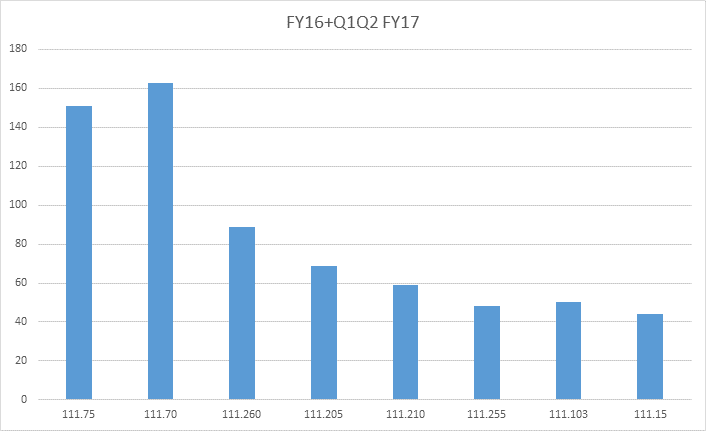
The most frequent sections of 21 CFR 111 to appear in FDA 483s were:
Specifications
- 111.75 - How a facility determines whether specifications are met
- 111.70 - Specifications that must be established
Master Manufacturing and Batch Production Records
- 111.205 - The requirement to establish a master manufacturing record
- 111.210 - What the master manufacturing record must include
- 111.255 - The requirement to establish a batch production record
- 111.260 - What the batch record must include
Written Procedures for Quality Control
- 111.103 - Requirements for written procedures
Sanitation Requirements
- 111.15 - Sanitation requirements applying to the physical plant and grounds
"The data from recent FDA inspections of dietary supplement facilities can help the industry efficiently allocate compliance resources to effectively communicate how facilities are complying with cGMP requirements when FDA inspectors visit," Dr. Zimmermann said.
Part 1 -- November 30
Veteran industry legal experts will provide an insider's view of the current cGMP enforcement landscape and strategies for compliance. AHPA staff will offer a detailed analysis of the association's repository of inspection data, which includes actual FDA 483 inspection reports, observation forms, and establishment inspection reports (EIRs).
A 20-30-minute Q&A session will answer attendees specific questions.
Part 2 -- December 14
An extended Q&A session will give attendees the opportunity to have their questions answered by the expert panel. Legal experts will then provide strategies for companies to effectively respond to an FDA 483 in order to avoid getting a Warning Letter. Attendees will also learn tips for conducting an FDA inspection.
This two-part webinar is designed to give companies time to reflect on the information presented in part one to ask follow-up questions during part 2.
Topics
- Own label distributors and contract manufacturers: What are the differences and what aspects of the cGMP are you responsible for during an inspection?
- FDA inspection practices and what can be done to prepare
- Detailed analysis of recent FDA inspection reports
- Effectively responding to an FDA 483 so as to avoid a Warning Letter
- FDA inspection do's and don'ts
- Answers to your questions during the Q&A session
Presenters
- Anthony L. Young, Esq., Partner, Kleinfeld, Kaplan and Becker, LLP / AHPA General Counsel
- Alan Feldstein, Esq., Of Counsel, Collins Gann McCloskey & Barry PLLC
- Merle Zimmermann, Ph.D., Chief Information Analyst, American Herbal Products Association (AHPA)