Since the first week of March, the Food and Drug Administration (FDA) and the Federal Trade Commission (FTC) have been issuing warning letters alleging that marketers promoted a wide variety of products with claims to prevent, treat, mitigate, diagnose or cure COVID-19.
AHPA has analyzed 135 COVID-19 related warning letters issued by either FDA or FTC or by the agencies jointly between March 6, 2020, and May 11, 2020, and made available in each agency’s coronavirus information center1 as of May 15, 2020.
“The regulated dietary supplement industry supports FDA and FTC using their authorities against any company or individual selling products making COVID-19 cure or prevention claims,” said AHPA President Michael McGuffin. “While research supports the use of certain herbs and dietary supplements to maintain healthy immune system responses, AHPA is not aware of evidence that would substantiate a claim that any dietary supplement is effective to prevent or treat COVID-19, and such a claim is not allowed under current U.S. law.”
Product types identified in warning letters
All but eight of the 135 analyzed warning letters identify one or more products or services as the subject of the letter’s recipient’s claims. The other eight letters were sent by FTC acting on its own and focused on COVID-19 claims in relation to business opportunities.
The most common subject of the claims in the remaining 127 warning letters is intravenous therapy, with 28 warning letters issued to date. Another 11 letters are directed to claims related to traditional Chinese medicine (TCM) products and therapies, and six letters name herbal products as the exclusive subjects of the COVID-19 claims. These and other product categories identified in at least five warning letters are shown in Figure 1.
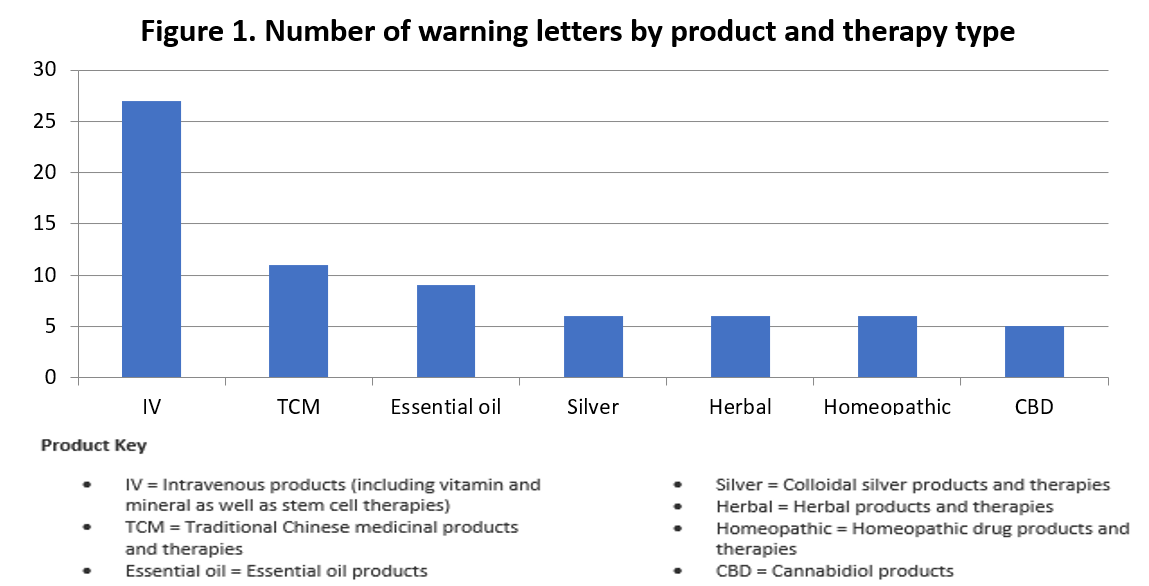
Figure 1 categorizes 70 warning letters by product or therapy type. An additional 32 letters identify a single claim subject (in order of frequency):
- vitamins (3 letters)
- air filters / sanitizers (3)
- honey (3)
- ozone (3)
- energy/vibrational therapies (2)
- hand sanitizer (2)
- kratom (2)
- acupuncture therapy (1)
- ayurvedic therapy (1)
- beverages (1)
- C60 mint strips (1)
- chiropractic therapy (1)
- chloroquine (1)
- copper (1)
- drug compounds (1)
- facial brushes (1)
- music (1)
- nasal spray (1)
- pet products (1)
- salt (1)
- zeolites (1)
Twenty-five other warning letters discuss multiple categories of products, for example a “viral protection kit” consisting of a nasal spray, suppository, and nebulizer, or a retail marketer making claims for liposomal vitamin c, a specific magnesium formula, and several products labeled to contain silver.
Claim sources and marketing channels identified in warning letters
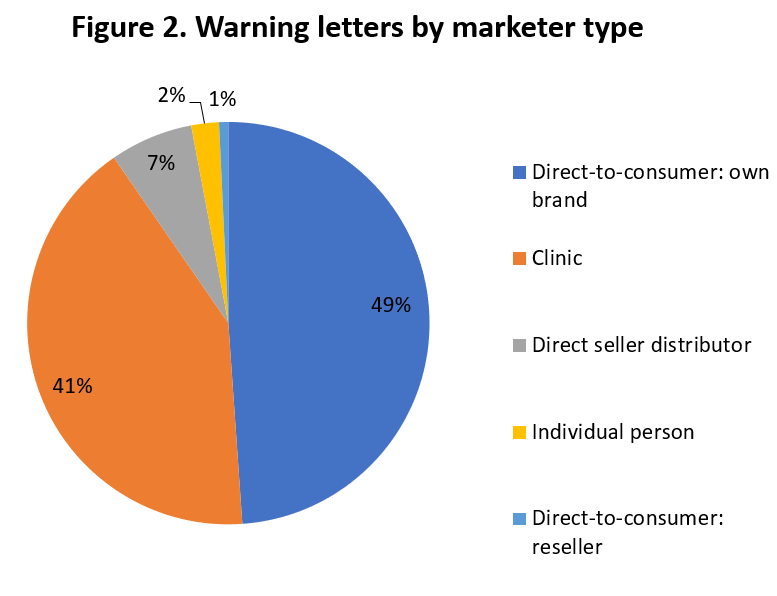 Warning letters appear to be primarily generated through agency review of website and social media marketing references. Only one letter mentions physical advertising at a retail location, and two others include mention of email newsletters. Letters indicate that claims made by direct-to-consumer marketers, clinics, direct seller distributors, and an occasional individual are receiving the agencies’ attention, as shown in Figure 2.
Warning letters appear to be primarily generated through agency review of website and social media marketing references. Only one letter mentions physical advertising at a retail location, and two others include mention of email newsletters. Letters indicate that claims made by direct-to-consumer marketers, clinics, direct seller distributors, and an occasional individual are receiving the agencies’ attention, as shown in Figure 2.
As shown in Figure 3 and articulated in some detail below, the 135 warning letters reviewed for this analysis were most often addressed to claims made on social media, followed by practitioner clinic websites and direct-to-consumer marketers.
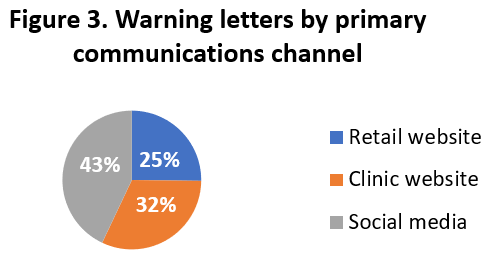
- Posts made on social media (including Facebook, Instagram, LinkedIn, Twitter, YouTube and other video sites, and blog posts) (58 warning letters; 43%)
- Sites offering clinical services and associated products (43 letters; 32%), and
- Retail websites offering direct sales of branded products (34 letters; 25%).
Social media posts frequently appear in a secondary role (in addition to the other two categories), with letters noting that they were repeating or directing consumers to places on retail websites and sites offering clinical services where claims were made. Some social media posts appear to be included because of the presence of hashtags such as “#COVID19” along with product information.
 Interagency cooperation
Interagency cooperation
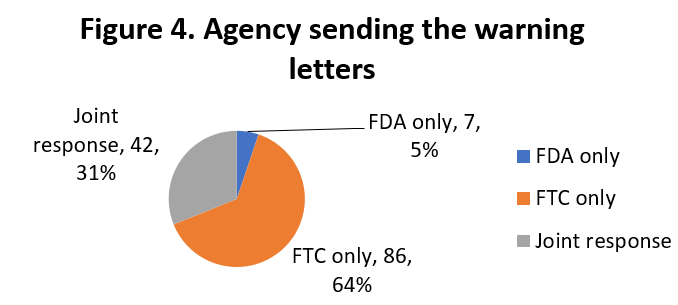
A and FTC are working together to send warning letters and each agency is also sending them individually, as indicated in Figure 4.
Limitations
The present analysis covered only data released by FTC and FDA prior to May 15, 2020. AHPA is aware that the agencies have released an additional 69 letters since the analysis was conducted, and further letters may still be made releasable and posted by the agencies after the publication of this article.
There are currently no products that are approved to treat or prevent COVID-19. AHPA has joined other dietary supplement industry organizations to vocally support efforts to enforce against products promoted with violative COVID-19 claims.
AHPA is continuing to compile these data as more warning letters are issued and will notify the industry about new information and trends. The best way to keep up to date on this information is to subscribe to AHPA Legal Alerts, which provide the latest enforcement news via email.
AHPA has additional resources available regarding COVID-19 at the AHPA COVID-19 Web Center, and AHPA also hosts related webinars, including the upcoming Free Webinar: Reopening Your Herbal Products Business -- COVID-19 & the “New Normal” (June 10) and previous events such as Webinar: COVID-19 Update for the Herbal Industry (Recorded March 26).
Analysis by Merle Zimmermann, Ph.D. (mzimmermann@ahpa.org), AHPA Chief Information Analyst.
For media inquiries, contact Haley Chitty (hchitty@ahpa.org), AHPA Director of Communications.